The Ministry of Health (MoH) has issued a public alert regarding the health product Bi Shu Ning, manufactured by Guang Zhou Wu Yang Pharmaceutical Co Ltd, which has been found to contain undeclared scheduled poisons, specifically Dexamethasone and Betamethasone. Testing by the Laboratory of Pharmacy Section, Department of Scientific Services revealed these adulterants, which pose serious health risks.
Long-term use of corticosteroids like Dexamethasone and Betamethasone can lead to elevated blood glucose levels, hypertension, cataracts, and increased infection risk, among other health issues. Users may also experience withdrawal symptoms, including fatigue and muscle pain, if they discontinue use after prolonged consumption.
The MoH emphasizes that there has been no approval for the importation or sale of Bi Shu Ning in Brunei, making its retail illegal. The public is urged to cease use of this product immediately and consult a healthcare professional if they experience adverse reactions.
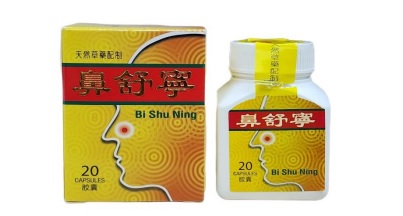
Selling this product, including online via platforms like Facebook, violates the Poisons Act 1956, with penalties reaching BND8,000 or six months’ imprisonment for offenders. If negligence endangers human life, fines may escalate to BND16,000 and up to 12 months’ imprisonment.
The Ministry calls upon the public to report any sightings of Bi Shu Ning to the Compliance and Licensing Section. For more information, contact the section at 2393301 extension 208, email pharmacy.enforcement@moh.gov.bn, or visit the Department of Pharmaceutical Services at Kg. Madaras, Mukim Gadong A. Talian Darussalam 123 is also available for assistance. – JAMES KON






